
Unexpected Outcomes & Complications
Bleeding
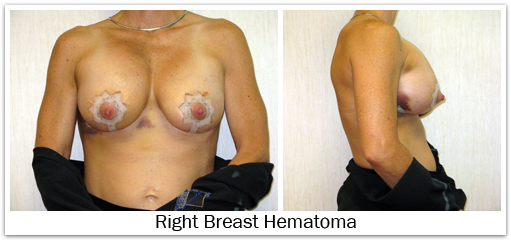
Bleeding is a potential risk of any operation. The likelihood of needing a blood transfusion from breast augmentation is extremely small. If post-operative bleeding were to occur, a collection of blood (hematoma) would form around the implant. This most likely will require re-operation to drain the blood, since proper healing will not occur if a hematoma remains around the implant. The risk of hematoma formation is approximately 1%-2%. It is important to avoid aspirin or anti-inflammatory medications for two weeks before surgery and about seven days following surgery, since these may increase the risk of bleeding. Excessive post-operative activity may also cause post-operative bleeding and hematoma formation. Non-prescription “herbs” and dietary supplements can increase the risk of surgical bleeding, so these should be discontinued two weeks prior to surgery as well.
Infection
Infection is unlikely to occur after breast augmentation, but can be very serious. Every effort is made to prevent an infectious complication, including use of intravenous antibiotics, rinsing the implant in antibiotic solution, placing antibiotics inside the pocket, minimizing implant contact with the skin, and providing postoperative oral antibiotics. Despite these measures, infections can still occur. Most infections appear within a few days to weeks after the operation, but they can occur at any time following insertion of a breast implant. Late infections of breast implants are rare occurrences. The risk of a severe infection following breast augmentation is less than 1%. Two types of acute infections could occur: an infection involving the soft tissue (skin, fat, breast tissue), or an infection involving the implant. Infections surrounding a breast implant are more difficult to treat than infections in normal body tissues; bacteria adhere to the implant surface where they are not effectively eliminated by the body’s immune response, or treated by antibiotic therapy. If an infection does not respond to antibiotics, the breast implant may have to be removed. After the infection is treated and the inflammation resolves, a new breast implant can usually be reinserted three to six months later. Sub-acute (sub-clinical) or chronic infections may be difficult to diagnose. In these situations, bacteria are present on the surface of the implant but do not cause any significant symptoms. The mild prolonged inflammation that results can lead to capsular contracture.
Breast Implant Failure
Neither saline nor silicone breast implants are considered permanent medical devices and can fail. Saline Failure – The sterile saline solution used to fill breast implants is i.v. fluid. It is a physiologic concentration (0.9% sodium chloride); the same saline which naturally comprises over two-thirds of our body. If a saline-filled implant were to fail, the saline will leak from the implant. The rate of saline leak depends upon the size and position of the hole. But generally, saline implants leak rather quickly. The saline which has leaked from the implant is now located around the implant shell, but within the capsule that surrounds the implant. The saline is safely absorbed by the body; so as the implant deflates the breast also shrinks in size. 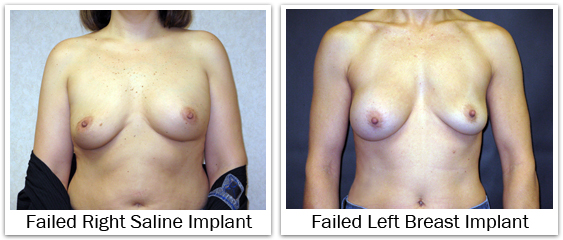
Silicone Gel Failure – If a silicone gel-filled implant should fail, the gel will leak form t he implant. The rate of gel leak depends upon the size of the hole and the cohesiveness of the silicone gel. The rate of silicone gel leak is extremely slow when compared to the rate of a saline leak. Any of the gel implants available today will demonstrate significantly less gel leakage when compared to their earlier counterparts. The moderately cohesive (responsive) gel implants will have a slightly greater tendency to leak than the highly cohesive (anatomic) gel implants. Any gel which has leaked from the implant is now located outside the implant shell, but inside he capsule that surrounds the implant. An intact capsule serves a protective role by preventing the gel from moving any further. Unlike saline, any leaking silicone gel is not absorbed, and therefore the breast remains stable in size. If you have heard the terms, “silent failure” or “silent rupture”, this is meant to describe a silicone gel implant failure in comparison to a saline failure. The saline implant failure is noticeable because the breast decreases in size, while the silicone failure may go unrecognized. The estimated failure rate for breast implants is about 1%-2% per year; this estimate is less predictive as the age of the implant increases. Published data for breast implant failure varies depending upon which device is being analyzed. For smooth round saline-filled implants, failure rates as low as 0.3% have been reported at three years post-op. When looking at all saline implants the approximate failure rate ranges from 2% at three years, to 7% at 5 years, to 10% at seven years post-op. Failure rates for silicone gel-filled implants are similar; 0.5% at two years, increasing to 10% at ten years post-op. In a five year analysis of silicone gel implants published by the FDA in 2011, the failure rate ranged from 7% to 13%. Failed or deflated implants cannot be repaired, and require an operation for removal and replacement.
Capsular Contracture
Capsule formation around a breast implant is normal and occurs in everyone following augmentation. The capsule is a thin fibrous layer that forms adjacent to the implant shell. If the implant has a smooth surface, the implant can move independently inside this capsule. If the implant has a textured surface, there is a possibility that the capsule will form within and amongst the depths and projections of the texturing, securing the implant shell so it cannot move. For reasons not completely understood, this capsule can tighten around the implant. This contraction of the capsule decreases the space available for the implant. If the contracture is more significant, the implant shell becomes folded on itself as the implant is forced to occupy a smaller space. Capsular contracture tends to make the breast more round as the surface area of the capsule decreases. In more significant cases, capsular contracture can change the size, shape, and/or the position of the implant, and the subsequent appearance of the breast. The breast implant becomes firmer as the contracture becomes more significant; it can even be painful in severe cases. The occurrence of capsular contracture is not predictable and contracture may occur soon after surgery or in years later, in one breast or both breasts, with saline implants or silicone implants, and with either implant placement under the breast gland or muscle. Every woman who has breast implants is at risk for capsular contracture. The exact cause of capsular contracture is not known but its formation is likely related to some instance or mechanism that causes excess scarring. Capsular contracture is likely to occur more frequently following subclinical infection, hematoma, and seroma. In addition, there is no one specific action or treatment to prevent capsular contracture from occurring. There is a general consensus that the risk of capsular contracture is higher when implants are placed in a subglandular position. The risk of capsular contracture might be slightly higher for implants placed through a periareolar incision, as opposed to an inframammary incision. And, silicone gel implants may have a slightly higher risk of capsular contracture than saline implants. 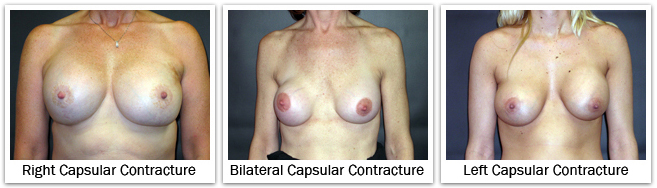 Breast capsules are classified by how the breast looks and feels. With a grade I capsule the breast looks and feels soft and natural. A grade II capsule causes the breast to feel a little firm, but the breast still looks soft and natural. A grade III capsule produces more firmness, and the breast looks abnormal. A grade IV capsule produces a hard breast with deformity and some associated pain. Grade III and grade IV contractures are more significant clinically, and are the ones that require an operation for treatment.
Breast capsules are classified by how the breast looks and feels. With a grade I capsule the breast looks and feels soft and natural. A grade II capsule causes the breast to feel a little firm, but the breast still looks soft and natural. A grade III capsule produces more firmness, and the breast looks abnormal. A grade IV capsule produces a hard breast with deformity and some associated pain. Grade III and grade IV contractures are more significant clinically, and are the ones that require an operation for treatment.
Scarring
Although every effort is made to make the incisions and resulting scars as minimal as possible, visible scars are possible with any operation. Incisions placed in the crease under the breast, and around the areola, tend to heal very well. The likelihood of a good scar starts with a good skin closure at the time of surgery; but each patient heals differently, and scars may heal better with appropriate post-operative attention. Scars may become lighter or darker than the adjacent skin coloration. Scars may widen under tension, and may thicken in response to increased inflammation. Exposure to UV radiation of the sun or a tanning bed, during the first year following surgery, may cause darkening of the scar or the skin around it. Your surgeon will provide suggestions to improve your healing after surgery.
Wrinkling or Rippling
The breast implant shell may wrinkle and/or ripple within the pocket; some of this is normal and expected. Many times these ripples can be felt through the skin, and in fewer instances these wrinkles may be visible. Wrinkling and rippling is often more pronounced with saline implants in comparison to silicone gel implants. Wrinkling and rippling is also more common in slender patients with minimal breast tissue and thin skin. When implants are placed in a subglandular position this wrinkling and rippling is often more visible, and can be more easily felt. For that reason, implant placement in a subpectoral position is preferred because it provides a greater thickness of tissue covering the breast implant, and therefore reduces wrinkling and rippling. 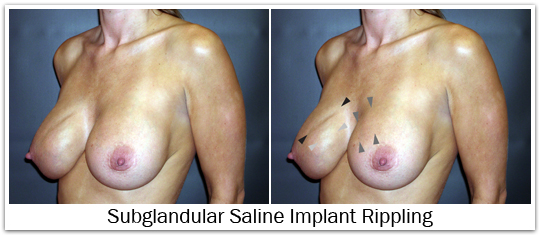
However, it is important to understand that the most common form of subpectoral placement uses a dual-plane pocket. A dual-plane pocket is actually a partial subpectoral and partial subglandular pocket; the medial and superior portions of the implant lie beneath the pectoralis major muscle, and the lateral and inferior portions of the implant lie beneath the breast gland and subcutaneous tissue. In a dual-plane pocket, visible and/or palpable rippling of the breast implant is more likely in the lateral and inferior portions of the breast where there is no muscle coverage.
Malposition
Implant malposition could refer to a situation where the implant is positioned too far medially, too far laterally, too high, or when the implant has dropped too low. If the pocket has been dissected too far laterally, the implant can easily slide sideways beyond the anterior axillary line producing excessive lateral fullness. This deformity is most apparent when the woman is lying on her back and the implant falls to the side. If the implant pocket has been dissected too far medially, a condition known as synmastia results; the implants touch in the middle, and normal cleavage is lost. If an implant remains totally submuscular, or if a very early capsular contracture forms, the implant may not drop and it persists in a high position on the chest wall producing excessive upper pole fullness. If the pocket is dissected too far inferiorly the implant will settle below the level of the inframammary crease producing a high-riding nipple position, loss of upper pole fullness, and sometimes a double bubble deformity. Implant malposition will often produce breast asymmetry. When this occurs re-operation is required to place the implant in a more natural position to restore proper shape. The risk of malposition requiring re-operation to reconstruct the inframammary fold is about 5%. 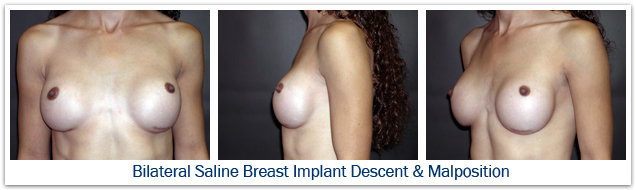
Synmastia
Synmastia is a form of ‘medial’ implant malposition. Synmastia occurs when both breasts meet or touch in the midline, rather than being positioned directly behind the breast mound. Synmastia occurs from inadvertent over-dissection of the medial pocket, and is more common when large implants are used; the shape of the patient’s rib cage and sternum, and weak tissues may also play a contributing role. It is easier for synmastia to occur following subglandular augmentation because there is no real medial anatomic barrier to limit medial dissection. Following a subpectoral augmentation, synmastia is less likely to occur because the implants are placed below the muscle, and the medial origins of the pectoralis muscle usually define the medial extent of pocket dissection. However, if significant release of the medial muscle origins was performed, the implants could move medially and synmastia it could develop. Synmastia is usually a problem that requires surgical correction to reconstruct and close the medial breast pocket.
Sensory Change
Alteration of skin and/or nipple sensation is not unusual following augmentation surgery, and can occur with any of the incision choices. Patients may also experience diminished or absent skin sensation, tingling, twinges, burning sensations, or shooting pains. Most of these sensory changes will resolve within several weeks to months following surgery. After several months, most patients have normal sensation. Partial or permanent loss of nipple and/or breast sensation may occur in a small percentage of individuals. The nerves that supply the breast skin and nipple are located near the lateral portion of the pocket created for the breast implant. These nerves may be damaged during surgery, or may be placed under pressure or stretch by the breast implant. Sensory changes may be more likely to occur with transaxillary (armpit incision) augmentation since this approach traverses the location of these nerves. An incision around the areola may carry a slightly increased risk of sensory change as well. 15-20% of women experience some numbness after breast augmentation, but most will resolve and return to normal within 12 months. The current risk for permanent sensory change following breast augmentation is 5%-10%. The addition of a breast lift to augmentation may increase the risk of sensory loss by an additional 5%.
Implant Animation
When the breast implant is located underneath the pectoralis major muscle (subpectoral position), the implant may become slightly flattened or move laterally and/or upward when that muscle is flexed with upper body workouts or exercises. This is referred to as “animation” of the implant. Breast implant animation is not harmful, but is sometimes undesirable because of the rather unnatural momentary shape change of the breast. This could be improved with re-operation, at the cost of less muscle coverage over the implant.
Breast Asymmetry
When comparing sides, it is very common for women to have one breast that is larger, wider, in a different position, or droops more than the other breast. Efforts can be made to minimize this asymmetry, but there will probably be some asymmetry that remains following augmentation surgery.
Mammography
Breast implants tend to be “radiographically opaque” on x-ray film – they appear more white because they block some of the passage of the x-ray beam. Silicone gel implants appear whiter than saline implants because they block more of the x-ray beam. Women with breast implants require special mammographic techniques to image their breast tissue. The presence of breast implants may make mammography more difficult, and the breast implant may obscure or limit the visualization of breast tissue or the detection of breast cancer. An ‘Eklund’ view mammogram is often performed to enhance breast tissue visualization. When using the Eklund technique the breast implant is pushed against the chest wall and the breast tissue is distracted forward, away from the implant, to improve the image of the breast tissue. Despite the use of displacement techniques, as much as one-third of a woman’s breast tissue may not be adequately visualized by mammography when breast implants are present. Subpectoral implants, compared with subglandular implants, may offer improved mammographic imaging of the breast tissue because the muscle functions as a barrier that partially separates the implant from the breast tissue, thereby minimizing overlap interference. The ratio of the amount of breast tissue to implant volume also plays a role in how easily the breast tissue is visualized by mammography. Breast tissue imaging will be reduced when there is a small amount of breast tissue and larger implants because it will be more difficult to separate the breast tissue away from the implant using displacement techniques. 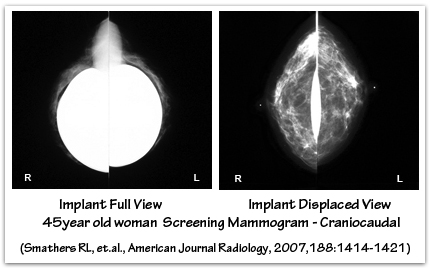
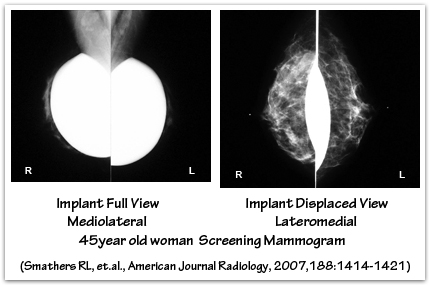
Saline breast implant rupture can occur from breast compression during mammography, although this very rare. Displacement techniques reduce compression of the implant, and the subsequent risk of capsule or device rupture. Saline implant failure during mammography is less likely to occur with smooth implants that are under the muscle, and in the absence of capsular contracture. Inform the mammography technologist that you have breast implants so that appropriate mammogram studies may be obtained. In some situations specialized mammography, ultrasound, and/or MRI studies may be necessary to evaluate breast lumps or the condition of the implants. If you are in your mid- to late-thirties, your surgeon may recommend a baseline mammogram before your breast augmentation surgery. A follow-up mammogram one year post-operatively is helpful to document any scarring or calcification changes for future reference.
Breast Cancer
The occurrence of breast cancer in women with breast implants has been evaluated in several large studies. In 2000, The National Cancer Institute (NCI) published their findings from a study of 13,500 women who had cosmetic breast augmentation surgery with saline or silicone gel implants prior to 1989. These women with breast implants were compared to women in the general population, as well as 4000 who had some other plastic surgical procedure other than breast augmentation. The average length of follow-up was more than 10 years. This NCI study found no association between breast implants and the risk of breast cancer. In June 1999, The Institute of Medicine (IOM) of the National Academy of Sciences published a report on the safety of silicone gel-filled breast implants. The IOM report was based upon a review of existing literature prior to 1999. The IOM did not find an increase in either primary or recurrent breast cancer in women with breast implants. Current information continues to support that there is not an increased risk of breast cancer in women who have either saline or silicone gel breast implants for cosmetic or reconstructive purposes.  When comparing women with breast implants to those without implants, breast cancer studies do not show any differences in the stage of disease at the time of diagnosis; and the prognosis is similar for women with, and without, implants. At the time of diagnosis, the sizes of tumors are equal in women with and without breast implants. Women with breast implants are diagnosed with palpable tumors more often than women without implants; this may be the result of breast tissue thinning, and the firmness of the implant against which the tumor can be felt. The lifetime risk of breast cancer is one in eight women. All women should perform a monthly self-examination of their breasts, have mammography according to American Cancer Society guidelines (annual screening mammography beginning at age 40, unless your risk is higher), and seek medical care if they notice a breast lump. The ability of mammography to image the breast tissue is limited by the presence of breast implants. However, there is no data to suggest that women with breast implants suffer from missed cancers or a significantly late diagnosis of breast cancer. Special techniques and extra images may be necessary to visualize the breast tissue when breast implants are present. Care must be exercised during breast biopsy procedures to avoid damaging the breast implant. More recently, there is thought to be a possible association between breast implants and anaplastic large cell lymphoma (ALCL), a rare form of non-Hodgkins lymphoma. ALCL is not a form of breast cancer.
When comparing women with breast implants to those without implants, breast cancer studies do not show any differences in the stage of disease at the time of diagnosis; and the prognosis is similar for women with, and without, implants. At the time of diagnosis, the sizes of tumors are equal in women with and without breast implants. Women with breast implants are diagnosed with palpable tumors more often than women without implants; this may be the result of breast tissue thinning, and the firmness of the implant against which the tumor can be felt. The lifetime risk of breast cancer is one in eight women. All women should perform a monthly self-examination of their breasts, have mammography according to American Cancer Society guidelines (annual screening mammography beginning at age 40, unless your risk is higher), and seek medical care if they notice a breast lump. The ability of mammography to image the breast tissue is limited by the presence of breast implants. However, there is no data to suggest that women with breast implants suffer from missed cancers or a significantly late diagnosis of breast cancer. Special techniques and extra images may be necessary to visualize the breast tissue when breast implants are present. Care must be exercised during breast biopsy procedures to avoid damaging the breast implant. More recently, there is thought to be a possible association between breast implants and anaplastic large cell lymphoma (ALCL), a rare form of non-Hodgkins lymphoma. ALCL is not a form of breast cancer.
Pregnancy and Breastfeeding
Both pregnancy and breast-feeding have a major hormonal and physiologic impact on the breast. Both can produce anatomic changes that affect the breast skin, the breast volume, and the breast shape. But no one is able to predict to what extent these changes will occur. Breast implants do not pose a risk to pregnancy, do not interfere with breast function, and do not affect a woman’s ability to breast-feed. The breast tissue behaves independent of the breast implant. Whatever breast tissue a woman has will respond to the hormonal changes of pregnancy, and breast-feeding, whether a breast implant is present or not. The amount of breast engorgement, skin stretch, and volume change varies with each woman; as does the degree of volume loss and drooping that may occur after pregnancy. If a woman has undergone breast augmentation before having children, she may experience less change in overall breast volume after pregnancy because the implants help maintain breast size and shape. However, additional surgery may be desired to improve the shape of the breast after pregnancy. Some women may chose to have a breast lift to tighten the skin envelope, while others may chose a slightly larger implant to fill out the loose breast skin. Other women may wish to wait until after having children, and then undergo breast augmentation surgery to restore the shape that has been lost following pregnancy and breast-feeding. Breast implants are used to restore the desired volume, and a breast lift can be added to restore shape and nipple position if necessary. Incision location probably has very little effect on a woman’s ability to breast-feed. Only if the breast gland is significantly divided will there be a potential change in a woman’s ability to breast feed; and most surgeons do not utilize such an approach. Most women, who have nursed prior to getting breast implants, have been able to successfully breast feed again after breast augmentation surgery. For women who have undergone breast augmentation before having children, they will retain whatever breast-feeding ability they had prior to surgery. It is important to understand that some women are incapable of breast-feeding, and they will remain so after breast augmentation surgery. If you choose to have breast implants after having children, it is important that your breasts return to a normal state before undergoing surgery. It is therefore best to wait at least 3 – 6 months following pregnancy and breast-feeding before your surgical procedure. Due to a risk of infection, you should not breast feed for 9 months after having your surgery. In addition, becoming pregnant immediately after your breast implant surgery may compromise your result due to the hormonal stimulation that occurs to the breast with pregnancy.
Long Term Changes
Subsequent alterations in breast shape may occur as the result of the stresses applied to the breast by the implant, aging, weight loss or weight gain, pregnancy, or other circumstances not related to breast augmentation. Breast sagging may normally occur as a result of breast volume loss or the loss of skin elasticity. Large breast implants may have a deleterious effect on long term breast shape by causing stretch marks, skin thinning, skin stretching, breast gland atrophy, and breast drooping.
Autoimmune and Connective tissue Diseases
More information to come
Platinum Toxicity
More information to come
ALCL
more information to come
Standard Anesthetic Risks
The administration of any medication has some amount of risk. Although every effort is made to minimize these risks, adverse reactions and side effects cannot always be prevented. Complications of anesthesia can range from slight dizziness or nausea, to more profound problems such as aspiration of gastric contents, anaphylaxis, the malfunction of anesthesia-related equipment, and human error. Although rare, hospitalization may be necessary to control and/or treat any potential complication that results from surgery and anesthesia. For a fair number of people, the thought of anesthesia is frightening. Some studies show that about 30% of patients are more afraid of general anesthesia than the operation itself, the post-operative pain, or the risk of infection or other significant complications. For a healthy individual without significant medical problems, the risk of a severe anesthesia-related medical complication or death is about 1 in 200,000. However, this risk is small enough to say that it is safer to have general anesthetic than get into an automobile (risk of death about 1 in 15,000). Breast augmentation is not risk free, but severe complications are rare.
Need for Additional Surgery
All surgical procedures involve risks and potential complications, and the results of surgery cannot be guaranteed. If your breast augmentation surgery does not turn out like you planned, it may be necessary to perform additional surgery to revise or improve your results, to remove or replace implants, or to treat one of the outcomes or complications listed above.
Additional Financial Cost
More information to come
Other Unexpected Outcomes
more information to come
NEXT TOPIC: Learn More in Breast Aug 301
What is the Gel-to-Shell Fill Volume Ratio?
Each round silicone gel-filled breast implant is comprised of a silicone elastomer shell which contains the silicone gel filling. Silicone gel breast implants are filled by the manufacturer with a moderately cohesive (responsive) silicone gel. If you look carefully at...
How Firm is a Saline Breast Implant?
During the consultation today, a patient asked me, "Aren't saline implants harder?" Well, it actually depends. Let me start by saying that if you have ever held a saline implant and a silicone gel implant side-by-side, you know that they feel completely different, and...
Why is a board certified plastic surgeon important for my breast augmentation?
I recently saw a woman in consultation for a failed saline breast implant. She had her breast augmentation performed seven years earlier by a surgeon who advertises like a plastic surgeon, but is not board certified in plastic surgery. And as far as I am aware, has...



